The Most Common Cause of Pathologic Hyperbilurubinemia in a Baby Is
Standing Education Activity
Neonatal jaundice or neonatal hyperbilirubinemia results from elevated total serum bilirubin (TSB) and clinically manifests as xanthous discoloration of the skin, sclera, and mucous membrane. In most cases, it is a mild, transient, and self-limiting condition and is referred to every bit "physiological Jaundice." Nevertheless, it is imperative to distinguish this from a more than severe form called "pathological Jaundice." Failure to place and care for this entity may upshot in bilirubin encephalopathy and associated neurological sequelae. This activity reviews the etiology, pathophysiology, evaluation, and management of neonatal jaundice and the office of the interprofessional team in the intendance of affected patients.
Objectives:
-
Place pathological jaundice and differentiate information technology from physiological jaundice.
-
Depict various causes of neonatal jaundice.
-
Review evidence-based direction options for neonatal jaundice.
-
Explicate how the interprofessional team can work collaboratively to prevent the potentially profound complications of neonatal jaundice by applying noesis about the presentation, evaluation, and management of this condition.
Access gratis multiple choice questions on this topic.
Introduction
Neonatal jaundice or neonatal hyperbilirubinemia results from elevated total serum bilirubin (TSB) and clinically manifests equally yellowish discoloration of the peel, sclera, and mucous membrane. The term jaundice derives from the French word "jaune," which means yellow. It is the virtually commonly encountered medical problem in the first two weeks of life and a common cause of readmission to the hospital later on birth.[1] Approximately 60% of term and 80% of preterm newborns develop clinical jaundice in the showtime week after birth.[2] In most cases, information technology is a mild, transient, and self-limiting condition and resolves without treatment referred to as "physiological jaundice." Even so, information technology is imperative to distinguish this from a more severe form called "pathological jaundice." Failure to place and treat this entity may outcome in bilirubin encephalopathy and associated neurological sequelae.
Unconjugated hyperbilirubinemia (UHB) is the cause of clinical jaundice in most neonates, simply some infants with jaundice have conjugated hyperbilirubinemia (CHB), which is always pathological and signifies an underlying medical or surgical cause. The etiology of pathological UHB and CHB is vast and varied. Preterm infants and those built-in with congenital enzyme deficiencies are particularly prone to the harmful furnishings of unconjugated bilirubin on the key nervous system.[iii][iv] Severe hyperbilirubinemia tin crusade bilirubin-induced neurological dysfunction (BIND) and, if non treated adequately, may pb to acute and chronic bilirubin encephalopathy.[5] Phototherapy and exchange transfusions are the mainstay of treatment of UHB, and a subset of patients too reply to intravenous immunoglobulin (IVIG). Handling of CHB is more complex and depends mainly on the etiology. Despite advances in intendance and management of hyperbilirubinemia, information technology remains a significant cause of morbidity and mortality.[half-dozen]
Etiology
There are two distinct types of Neonatal hyperbilirubinemia.
Unconjugated Hyperbilirubinemia(UHB) or Indirect Hyperbilirubinemia
Unconjugated hyperbilirubinemia is the more common blazon and is either physiological or pathological. Physiological jaundice accounts for 75% of neonatal hyperbilirubinemia and results from a physiological alteration in neonatal bilirubin metabolism. Healthy adults have a normal TSB level of less than 1mg/dl in contrast to neonates, where TSB levels are physiologically higher. Even in good for you full-term newborns, in that location is an increased bilirubin load owing to increased blood-red blood cells (RBC) mass and a decreased RBC lifespan. Clearance of bilirubin is also compromised due to impaired activity of uridine diphosphate glucuronosyltransferase (UGT), the enzyme needed for bilirubin conjugation. The UGT enzyme in a newborn has an activity of most 1% of the adult level.[seven] Moreover, these infants besides have increased enterohepatic circulation, further contributing to elevated TSB levels. Physiological jaundice typically appears later on 24 hours of age, peaks at around 48-96 hours, and resolves past two to iii weeks in full-term infants.[two]
Jaundice is considered pathological if it presents on the first day of life, TSB is more than the 95th centile for age based on age-specific bilirubin nomograms, levels rising by more than five mg/dL/day or more than 0.2 mg/dL/hour, or jaundice persists beyond ii to 3 weeks in total-term infants.[8]
Based on the mechanism of bilirubin top, the etiology of unconjugated hyperbilirubinemia tin exist subdivided into the following 3 categories:
Increased Bilirubin Product
Immune-mediated hemolysis - Includes blood group incompatibilities such as ABO and Rhesus incompatibility.
Non-immune mediated hemolysis - includes RBC membrane defects similar hereditary spherocytosis and elliptocytosis; RBC enzyme defects like glucose-6-phosphate dehydrogenase (G6PD) deficiency; pyruvate kinase deficiency; sequestration similar cephalohematoma, subgaleal hemorrhage, Intracranial hemorrhage; polycythemia, and sepsis.
Decreased Bilirubin Clearance
Crigler-Najjar blazon I & Ii, and Gilbert syndrome.
Miscellaneous Causes
Other miscellaneous etiologies include the infant of a mother with diabetes, built hypothyroidism, drugs like sulfa drugs, ceftriaxone, and penicillins, Intestinal obstruction, pyloric stenosis, chest milk jaundice, breastfeeding jaundice.
Exaggerated hemolysis, either allowed or non-immune mediated, is the most common cause of pathological hyperbilirubinemia in newborns. Immune-mediated hemolysis is seen with blood grouping incompatibility such every bit ABO/RH incompatibility and leads to hemolytic disease of newborns (HDN). In HDN, due to ABO incompatibility, preformed maternal anti-A and anti-B antibodies of immunoglobulin (Ig) G bracket cross the placenta and cause hemolysis and UHB in newborns with blood type A, B, or AB. Although the straight Coombs exam is used to aid diagnosis, the sensitivity and positive predictive value for predicting astringent UHB are low.[ix] ABO incompatibility between female parent and fetus exists in about 15% of pregnancies, just HDN due to ABO incompatibility is seen only in 4% of newborns with ABO incompatibility.[10]
In Rhesus (Rh) incompatibility, an Rh-negative female parent who has been previously exposed to Rh-positive RBCs unremarkably from a previous pregnancy or miscarriage, becomes sensitized and develops antibodies against Rh antigen. Initially, sensitization produces IgM antibodies that can not cantankerous the placenta. Notwithstanding, during subsequent pregnancies, the antibiotic class switch produces IgG antibodies which can cross the placenta, causing RBC hemolysis in the fetus with Rh-positive blood. The Rh antigen is very immunogenic, and the resultant HDN is usually severe, oft leading to hydrops in fetuses or severe UHB in newborns. The American College of Obstetricians and Gynecologists (ACOG) has recommended that all Rh-negative pregnant women receive anti-D allowed globulin at 28 weeks of gestation and again following commitment if the infant is Rh-positive/unknown.[xi]
Not-immune causes of UHB include RBC enzyme defects, RBC membrane defects, hemoglobinopathies, sepsis, sequestration, and polycythemia. The glucose-half-dozen phosphatase dehydrogenase (G6PD) enzyme deficiency is the most common RBC enzyme defect and is transmitted equally an X-linked recessive trait. G6PD protects RBCs against oxidative harm by generating NADPH (nicotinamide adenine dinucleotide phosphate hydrogenase) from NADP (nicotinamide adenine dinucleotide phosphate). When exposed to oxidant stressors like illness, certain medications, dyes, and foods like fava beans, G6PD scarce RBCs are hemolyzed, causing anemia and hyperbilirubinemia. More than 200 different types of mutations are known to cause G6PD deficiency.[12] The clinical presentation varies depending on the variant, and some newborns may develop severe hyperbilirubinemia and bilirubin encephalopathy. Pyruvate kinase deficiency(PKD) is another enzyme deficiency that causes hemolysis and may present as UHB in newborns. It is an autosomal recessive(AR) disorder caused by a defect in Adenosine triphosphate (ATP) synthesis mechanism. In PKD, RBCs and, in item, young RBCs accept shortened life span resulting in anemia and UHB.[xiii]
UHB due to RBC membrane defects includes hereditary spherocytosis (HS) and hereditary elliptocytosis (HE). HS, besides known equally Minkowski Chauffard illness, is the near common RBC membrane defect caused by mutations in RBC membrane proteins.[14] About cases are transmitted equally an autosomal dominant (AD) trait and tin nowadays in the neonatal flow with UHB.[xv] Hereditary elliptocytosis is some other type of RBC membrane defect that is by and large asymptomatic simply rarely does cause UHB in the neonatal catamenia.[sixteen] Most cases are transmitted as Ad traits caused by mutations in RBCs structural membrane protein. The elliptical-shaped RBCs in HE are trapped in the spleen leading to extravascular hemolysis and elevated TSB.
RBC sequestrations from cephalohematoma, subgaleal hemorrhage, and Intracranial hemorrhage are also important causes or run a risk factors for UHB in the neonatal period due to increased bilirubin load. Polycythemia is some other entity associated with an increased risk of UHB in newborns. Clinical weather associated with polycythemia are intrauterine growth restriction (IUGR), infant of diabetic mothers (IDM), large for gestational age (LGA), maternal smoking, loftier altitude, twin to twin transfusion, and placental transfusion (delayed cord clamping/umbilical string milking). Studies accept shown that placental transfusion reduces the incidence of postnatal anemia and leads to improved neurodevelopmental outcomes among term and preterm infants.[17][xviii] This practice has gained popularity, but at the aforementioned time, it may also increase the risk of hyperbilirubinemia.[19][twenty]
Indirect hyperbilirubinemia due to decreased bilirubin clearance unremarkably results from quantitative or qualitative defects in the uridine diphosphate glucuronosyltransferase (UGT) enzyme. Gilbert syndrome, Crigler–Najjar syndrome blazon one, and Crigler–Najjar syndrome type 2 are iii prototype disorders resulting from an abnormality in the UGT enzyme. Gilbert syndrome is the most common of these and results from a mutation in the UGT1A1 factor resulting in decreased UGT production leading to unconjugated hyperbilirubinemia.[21] Gilbert syndrome typically presents as balmy jaundice at times of stress in the absence of hemolysis or liver dysfunction.[22] Presentation in the neonatal menses is rare and is ordinarily associated with G6PD.[3] Crigler-Najjar syndrome type one is an AR disorder resulting from a consummate absence of UGT activity. Affected patients nowadays with severe hyperbilirubinemia in the first days of life, often leading to bilirubin encephalopathy. Patients with Crigler-Najjar syndrome type 2 retain some of the action of UGT enzymes. As such, the TSB levels are not that high, and patients rarely develop bilirubin encephalopathy.[23]
Breast milk jaundice and breastfeeding jaundice are two other common etiologies of UHB in newborns. Breastfeeding jaundice, likewise known equally breastfeeding failure jaundice, occurs in the kickoff week of life and is due to inadequate intake of chest milk leading to dehydration and sometimes hypernatremia.[7] Breastfeeding failure leads to decreased intestinal move and decreases the emptying of bilirubin in the stool or meconium. Chest milk jaundice occurs late in the first calendar week, peaks in the 2nd, and normally resolves by two weeks of age. It is thought to exist mainly due to inhibition of the UGT enzyme by pregnanediol and deconjugation of conjugated bilirubin in the intestines past beta-glucuronidase present in breast milk.[24][25]
Other miscellaneous causes of UHB include IDM, gastrointestinal obstruction, congenital hypothyroidism, certain medications. IDMs often accept polycythemia which is mainly responsible for the increased incidence of jaundice in these infants.[26] UHB in congenital hypothyroidism is related to decreased hepatic uptake of bilirubin, impaired UGT activeness, and sluggish gut movement. Gastrointestinal obstruction promotes increased bilirubin recycling by augmenting the enterohepatic circulation. When used in the neonatal period, certain medications may also worsen UHB by displacing bilirubin from albumin, affecting albumin binding.[27] Sepsis can also predispose a newborn to UHB by causing oxidative impairment to RBCs, increasing bilirubin load.[28]
The majority of infants with clinical UHB have a combination of two or more factors discussed earlier. Furthermore, certain recognized gamble factors predispose an infant to jaundice. These run a risk factors comprise prematurity, a history of jaundice in previous siblings requiring phototherapy, Asian ethnicity, male gender, and exclusive breastfeeding.[2] Lastly, UHB in premature infants presents as a special scenario. It is believed that preterm infants take an increased gamble of bilirubin encephalopathy and kernicterus in addition to being at a college of jaundice. Yet, at present, there is a dearth of data on the magnitude of the problem as well as consensus guidelines on the management of UHB in preterm infants.[29][30] The TSB threshold for initiation of phototherapy and criteria for exchange transfusion is besides not clear in this population. Bilirubin is an antioxidant and may have a physiological part in neonates.[31][32] Keeping TSB levels low by aggressive treatment in preterm infants may reduce the antioxidant level and potentially worsen the retinopathy of prematurity. Reduced antioxidant status is also associated with chronic lung disease and neurological injury. As such, treatment of UHB in this population is a challenging task in the absence of prove-based guidelines.[29]
Conjugated Hyperbilirubinemia(CHB) or Direct Hyperbilirubinemia
Conjugated hyperbilirubinemia, too referred to equally neonatal cholestasis, is characterized past elevation of serum conjugated/straight) bilirubin (> 1.0 mg/dL) and is due to impaired hepatobiliary function. Distinguishing CHB from UHB is critical considering cholestatic jaundice/CHB is about always pathologic and warrants prompt evaluation and treatment.[33]
The causes of neonatal cholestasis/CHB are extensive and tin can exist classified into the following categories:
Obstruction of biliary flow: Biliary atresia, choledochal cysts, neonatal sclerosing cholangitis, neonatal cholelithiasis
Infections: CMV, HIV, rubella, herpes virus, syphilis, toxoplasmosis, urinary tract infection (UTI), septicemia
Genetic causes: Alagille syndrome, blastoff-i anti-trypsin deficiency, galactosemia, fructosemia, Tyrosinemia type one, cystic fibrosis, progressive familial intrahepatic cholestasis (PFIC), Aagenaes syndrome, Dubin-Johnson syndrome, Bile acid synthesis disorders(BSAD)
Miscellaneous: Idiopathic neonatal hepatitis, parenteral nutrition induced cholestasis, gestational alloimmune liver illness/neonatal hemochromatosis, hypotension,
Biliary atresia (BA) is the most mutual crusade of conjugated hyperbilirubinemia in infants.[34] The incidence of BA varies from region to region. Information technology is reported at a frequency of 1 in 6000 alive births in Taiwan, the region with the highest incidence. In the United States, information technology has an incidence of around 1 in 12,000 alive births.[35] The etiology of BA is not well understood, but genetic factors along with viral infection, toxins, chronic inflammatory and autoimmune injury to bile ducts seem to play a role in its pathogenesis. The disease involves both intra-hepatic and extra-hepatic bile ducts and classically presents around ii to 4 weeks of life with stake stools and jaundice. The initial evaluation is by ultrasonography that may show an absent gallbladder and the archetype "triangular cord" sign.[36] Early diagnosis is critical to maximizing the response to a Kasai functioning (hepatic portoenterostomy).[37] If the surgery is delayed by 90 days of life, less than 25% of patients are reported to answer, compared to surgery performed within lx days when more than lxx% of patients will establish adequate bile flow.[38]
Choledochal cysts involve dilation of the intrahepatic and actress-hepatic bile duct. Ultrasonography tin detect cysts with normal or dilated intrahepatic bile ducts as opposed to sclerosed ducts in biliary atresia. However, cystic biliary atresia may resemble choledochal cysts.[39] Neonatal sclerosing cholangitis (NSC) is a rare form of cholangiopathy that ofttimes presents in infancy with CHB, hepatosplenomegaly, pale stools, and loftier serum gamma-glutamyltransferase activeness (GGT).[40] Neonatal cholelithiasis is too a rare entity that tin crusade significant straight hyperbilirubinemia in neonates.[41]
Cytomegalovirus (CMV) is the nigh common congenital infection that manifests in various ways. Most infected newborns are asymptomatic, only hepatomegaly and CHB are the well-nigh prominent feature of hepatic involvement.[42] Syphilis, toxoplasmosis, herpes, and rubella should be included in the differential diagnosis of neonatal cholestasis, peculiarly when other stigmata of congenital infection similar growth restriction, coagulopathy, skin rash, and thrombocytopenia are present. Careful evaluation of maternal history along with specific serologies and culture would aid the diagnosis. UTI is too a significant crusade of CHB in neonates, and a urine culture should be included equally part of diagnostic evaluation. Microcirculatory changes in the liver, a straight effect of bacterial products, and toxins released by bacteria are thought to be the possible mechanism of cholestasis in patients with UTI.[43]
Alagille syndrome (ALGS) is an AD disorder caused by mutations in JAG1 or NOTCH2 genes leading to a lack of interlobular bile ducts.[44] With an incidence of 1 in thirty,000 alive births, ALGS is the virtually common cause of familial intrahepatic cholestasis.[33] Characteristic clinical features in add-on to cholestasis are butterfly vertebrae, congenital heart defect (almost commonly peripheral pulmonic stenosis), kidney involvement, dysmorphic features (broad forehead, small pointy chin), and posterior embryotoxic of the heart. GGT levels are elevated out of proportion, often up to xx times their normal value. Interestingly, CHB in patients with ALGS may resolve with historic period.[45] Few patients with cystic fibrosis (CF) can present with features of cholestasis because of abnormal bile that plugs the bile ducts.[46] In developing nations where newborn screening with immunoreactive trypsinogen is unavailable, neonatal cholestasis may exist the first clue to the diagnosis.
Blastoff-one-antitrypsin deficiency is the most mutual genetic crusade of cholestatic and may mimic biliary atresia in early infancy. Accumulation of anti-trypsin polymers in the endoplasmic reticulum of hepatocytes of a patient with the PiZZ genotype leads to apoptosis of hepatocytes, ultimately resulting in cholestasis and cirrhosis later in childhood.[47] Equally with ALGS, cholestasis may also amend with age equally with ALGS. Galactosemia, fructosemia, and tyrosinemia type 1 are a few of the inborn errors of metabolism known to cause cholestasis in neonates. Newborns with galactosemia nowadays with cholestatic jaundice, cataracts, hepatomegaly, failure to thrive, renal tubular acidosis, and Escherichia coli sepsis after the ingestion of galactose from milk.[48] Galactose-1-phosphate uridyl transferase (GALT) deficiency leads to the accumulation of toxic galactose metabolites in multiple organs. The presence of reducing substances in urine suggests galactosemia, and GALT activity in the liver or erythrocytes confirms the diagnosis. Neonatal cholestasis may be a presenting feature in hereditary tyrosinemia blazon 1, another AR disorder acquired by deficiency of enzyme fumarylacetoacetate hydroxylase. Other features of this disorder are renal Fanconi syndrome, hepatomegaly, coagulation abnormality, and the hazard of hepatocellular carcinoma in untreated patients.[49]
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous grouping of 3 genetic disorders that present with cholestasis. They are related to mutations in one of the genes involved in canalicular hepatobiliary ship.[50] Types 1 and 2 usually manifest in the neonatal period, while type 3 presents afterwards in infancy. Affected patients frequently develop cirrhosis and finish-stage liver disease during childhood. GGT level is normal in types 1 and 2 and elevated in blazon 3 patients. PFIC1 is acquired by a mutation in the ATP8B1 gene, which encodes FIC1 protein, whereas PFIC2 is caused by a mutation in the ABCB11 cistron, which encodes for the bile salt excretory protein (BSEP). PFIC 3 is caused by a mutation in the ABCB4 gene, which encodes for the multi-drug resistant-3 protein (MDR3).[51] Aagenaes syndrome, too known equally lymphedema cholestasis syndrome (LCS), is another type of idiopathic familial intrahepatic cholestasis syndrome characterized past neonatal cholestasis and lymphedema in lower extremities. It is transmitted every bit an AR trait and is generally seen in individuals of Norwegian descent.[52] Dubin-Johnson syndrome (DJS) is a rare AR disorder caused past a mutation in the ABCC2 factor, which codes for a non-biliary ion transporter in the liver. A unique feature of DJS is the presence of black liver and excretion of coproporphyrin 1 in urine.[53] Bile acid synthesis disorder (BASD) results from a deficiency of ane of the enzymes involved in synthesizing bile acids from cholesterol. BASDs are an uncommon cause of cholestasis, but many of these are curable with medical therapy alone.
Parenteral nutrition-associated cholestasis (PNAC) is an important iatrogenic crusade of cholestasis recognized most commonly in preterm infants managed with parenteral nutrition (PN). PNAC is present in most 20% of neonates who have received PN for more than 2 weeks.[54] Elapsing of PN utilise and intestinal failure are ii independent hazard factors for PNAC. The mechanism is not entirely clear and is probably multifactorial.[55] Aberrant bile common salt metabolisms due to prematurity and harmful furnishings of components of PN are thought to be the main culprit. Other factors such as sepsis, and necrotizing enterocolitis, appear to potentiate liver injury.[56] Gestational alloimmune liver disease (GALD), which causes almost all neonatal hemochromatosis cases, is a fulminant alloimmune disorder and results from intra-hepatic and actress-hepatic fe degradation resulting in liver failure.[57]
In GALD, maternal IgG immunoglobulin against fetal hepatocytes crosses the placenta causing complement-mediated damage to fetal hepatocytes. Patients present with signs of liver failure in the form of hypoglycemic, coagulopathy, hypoalbuminemia, cholestatic jaundice, edema, and elevated liver enzymes. The run a risk of recurrence in subsequent pregnancies is almost ninety%, and GALD can result in fetal or neonatal deaths.[58] The term idiopathic neonatal hepatitis is used when the etiology of neonatal cholestasis cannot be ascertained after an extensive diagnostic workup. The size of this entity is shrinking with advancements in newer diagnostic tools, with more and more than causes of neonatal cholestasis beingness identified that were originally labeled as idiopathic neonatal hepatitis.[38]
Epidemiology
Unconjugated hyperbilirubinemia is a commonly encountered problem in the neonatal catamenia. It is estimated that nigh 60% of term and 80% of preterm newborns volition present with clinical jaundice with TSB >five mg/dl.[ii] However, only about x% of newborns are estimated to require phototherapy for jaundice.[59] Physiological jaundice is considered the most frequent cause of clinical jaundice afterwards the first day of life, accounting for approximately 50% of cases.[sixty] Around xv% of breast-fed infants will develop UCH lasting for more than three weeks.[61]
Simply a minority of infants with neonatal jaundice have a pathological cause of jaundice. The incidence of severe hyperbilirubinemia, defined as TSB>25 mg/dl, is about i in 2500 live birth. Among these, ABO incompatibility followed by G6PD deficiency is the most frequently identified cause identified.[62] Newborns with Southeast and Far East Asian ancestry have higher recorded TSB levels than their White and African counterparts.[63][64] Neonatal jaundice besides appears to exist more common in people living at high altitudes and those living around the mediterranean sea, especially in Greece.[65][66]
The incidence of acute bilirubin encephalopathy is seen at a rate of approximately 1 in x,000 live births, whereas the incidence of chronic bilirubin encephalopathy is lower, with an estimated incidence of ane in 50,000 to 100,000 alive births.[67] However, in developing nations, the estimated occurrence of kernicterus is much higher.[68]
Conjugated hyperbilirubinemia is much less mutual compared to UCH, with a frequency of effectually i in 2500 term infants.[69] The most common identifiable crusade of cholestatic jaundice in the neonatal period is Biliary atresia accounting for about 25% to 40% of all cases, followed past infections and TPN-induced cholestasis.[33][lxx][33] It is estimated that 60% to 70% of patients with BA will eventually crave liver transplantation in childhood, and BA remains the about common indication for a pediatric liver transplant.[71]
Pathophysiology
Bilirubin is produced from the catabolism of heme, a breakdown product of hemoglobin, in the reticuloendothelial system (RES). First, heme is converted to biliverdin, releasing fe and carbon monoxide via the action of enzyme heme oxygenase.[72] Biliverdin is so converted to bilirubin past the enzyme biliverdin reductase. This unconjugated bilirubin is hydrophobic and is transported in circulation to the liver bound to albumin, where it is conjugated with glucuronic acid in the shine endoplasmic reticulum by the enzyme uridine diphosphate-glucuronosyltransferase (UGT). Conjugated bilirubin is water-soluble and is then excreted in bile and into the gastrointestinal (GI) tract, where it is by and large excreted in feces later being metabolized by abdominal bacterial flora. Some of the conjugated bilirubin is deconjugated in the GI tract by the action of beta-glucuronidase and is reabsorbed through the enterohepatic circulation.[73]
Newborn infants have college TSB levels than adults owing to higher hemoglobin levels at birth, forth with a shorter RBC life span and limited conjugating ability of the newborn liver.[74] As such, total-term newborns normally have pinnacle serum bilirubin concentrations of five to 6 mg/dl compared to adult levels of <1 mg/dl. Pathological jaundice in neonates is related to increased production of bilirubin in RES, impaired hepatic uptake, deficient conjugation of bilirubin, and/or enhanced enterohepatic circulation of bilirubin.[72]
In astringent hyperbilirubinemia, unbound and unconjugated bilirubin crosses the blood-brain bulwark and binds to the brainstem, hippocampus, cerebellum, globus pallidus, and subthalamic nuclei.[2] At the cellular level, bilirubin inhibits certain mitochondrial enzymes, inhibits Deoxyribonucleic acid and protein synthesis, induces breaks in Deoxyribonucleic acid strands, and hampers phosphorylation.[75] Bilirubin besides impairs tyrosine uptake and alters the normal operation of N-methyl-D-aspartate–receptor ion channels.[76][77] These mechanisms are implicated in the pathogenesis of bilirubin toxicity that clinically manifests as bilirubin-induced neurologic dysfunction (Demark) and bilirubin encephalopathy. The duration of exposure to bilirubin and the corporeality of bilirubin in the brain determines the severity of encephalon damage. However, the TSB level does not correlate well with bilirubin toxicity in the absence of hemolysis.[72] Preterm infants are even more vulnerable to the toxic furnishings of gratis unconjugated bilirubin. This is in part related to comparatively lower serum albumin level, CNS immaturity, and concurrent comorbidities like intraventricular hemorrhage, periventricular leukomalacia, sepsis, necrotizing enterocolitis, and bronchopulmonary dysplasia.[68]
Conjugated hyperbilirubinemia results from abnormalities in the uptake, metabolism, transport, and/or excretion of bile salts and bilirubin.[78] These abnormalities increase bile acid in the liver that promotes the proliferation of bile ducts and fibrosis. Bile acid is also responsible for inflammation and apoptosis of hepatocytes culminating in hepatocellular injury and cirrhosis.[79] Deficient bile secretion in cholestasis results in malabsorption of fat and fat-soluble vitamins that ofttimes leads to failure to thrive with vitamin A, D, E, and One thousand deficiencies.[fourscore]
Histopathology
The term Kernicterus denotes xanthous staining of deeper brain nuclei seen on autopsy specimens on infants with severe unconjugated hyperbilirubinemia. The histopathologic features seen on these autopsies include nuclei that have undergone pyknosis, the presence of vacuolation in the cytoplasm, and fading of the Nissl substance.[81]
A liver biopsy is often needed for making a definitive diagnosis of cholestasis. It may help differentiate Biliary atresia from idiopathic neonatal hepatitis. Histopathological features of BA include the expansion of the hepatic portal tracts with edema, fibro-dysplasia, bile ductular proliferation, and bile plugs in the ductal lumen. Multinucleate giant cells and hemopoiesis are other features often seen on histopathologic exams of cholestatic liver samples.[82] Although not diagnostic of whatever disorder, the prominence of hepatic erythropoiesis is seen more ofttimes in cholestasis of infectious etiology. The pathognomonic histopathological features of other cholestatic disorders include periodic acid- Schiff (PAS)-positive granules in alpha-1 antitrypsin deficiency, paucity of bile ducts in Alagille syndrome, necrosis, and inflammation effectually duct seen in sclerosing cholangitis.[83]
Amongst familial causes of cholestasis, canalicular cholestasis with a marked absence of ductular proliferation and isolated periportal biliary metaplasia of the hepatocytes is ordinarily seen in PFIC1 patients. In PFIC2 patients, the histopathology is similar except that altered liver architecture and extensive lobular and portal fibrosis with inflammation are more common.[51]
History and Physical
The evaluation of the neonate with jaundice starts with a detailed history, including birth history, family history, the onset of jaundice, and maternal serologies. Color of stool and urine presence of pruritis should exist assessed for infants evaluated for jaundice and may provide a inkling to the blazon of jaundice. The American Academy recommends universal screening of all newborns for jaundice and identifying risk factors for developing severe hyperbilirubinemia.[8] Major take a chance factors in newborns over 35 weeks gestation include pre-discharge bilirubin in the high-risk zone, jaundice observed in the outset 24 hours, claret group incompatibility, gestational age 35 to 36 weeks, a previous sibling who received phototherapy, cephalhematoma or significant bruising, exclusive breastfeeding and e Asian race. Prematurity is also a known risk factor for developing severe hyperbilirubinemia.[84] Small-scale risk factors are serum bilirubin in the high intermediate-range, macrosomic babe of a diabetic mother, polycythemia, male person gender, and maternal age older than 25 years.[8]
To assess for jaundice, newborns should ideally exist examined in daylight. However, the clinical assessment may be unreliable, especially if a newborn has received phototherapy or has dark pare.[85] Therefore clinically significant jaundice should always be confirmed with a TSB or transcutaneous bilirubin. A focused physical examination to place the cause of pathologic jaundice should be performed. Evaluation for pallor, petechiae, cephalhematoma, subgaleal bleed, extensive bruising, hepatosplenomegaly, weight loss, signs of dehydration needs to be washed. All infants with jaundice should also be assessed for signs and symptoms of bilirubin encephalopathy that includes poor feeding lethargy, altered slumber, aberrant tone, or seizures. It is, however, important to note that up to 15% of neonates with kernicterus are clinically asymptomatic in the newborn period.[72] Equally discussed in prior sections, certain etiologies of neonatal cholestasis have multi-system involvement. These signs should be looked for during concrete exams that may often provide a clue to diagnosis and aid in directing specific work-upwardly.
Evaluation
Diagnosis of Unconjugated Hyperbilirubinemia
Bilirubin levels tin be assessed using a transcutaneous measurement device or blood samples for total serum bilirubin. Transcutaneous estimation of bilirubin reduces the frequency of blood tests, only its utility is limited in infants with nighttime skin and following phototherapy use.[86][87] The serum level should be measured when the transcutaneous bilirubin (TcB) level exceeds the 95th percentile on the transcutaneous nomogram or 75% of the TSB nomogram for phototherapy. Another limitation of relying on TcB is the inability to detect the straight fraction of bilirubin required for diagnosing neonatal cholestasis.
Recommended workup for identifying a hemolytic illness as the cause of unconjugated hyperbilirubinemia include maternal/neonatal blood blazon, Coombs test, complete blood jail cell (CBC), reticulocyte count, blood smear, and G6PD. Serum albumin should always be checked, specially if TSB level approaches near the substitution transfusion levels, as information technology is considered a surrogate mark for complimentary bilirubin. Free bilirubin is the fraction responsible for bilirubin-induced toxicity.[88] Bilirubin-albumin ratio(B/A) ratio is, therefore, an additional tool that may predict the risk of kernicterus and may serve every bit an alternative guide to exchange transfusion.
Radiographic imaging is usually not required for most cases of UCH. Magnetic resonance imaging (MRI) findings take high sensitivity for bilirubin encephalopathy, with posteromedial borders of the globus pallidus being the most sensitive brain region for detecting indicate changes. Infants with bilirubin encephalopathy demonstrate hyperintense signals on T1-weighted sequences in the acute stage that eventually becomes hyperintense on T2-weighted sequences equally the disease evolves. Magnetic resonance spectroscopy(MRS) shows increased levels of glutamate and decreased levels of N-acetyl-aspartate and choline.[89] However, the absence of these findings does not exclude the take a chance of chronic bilirubin encephalopathy.
Diagnosis of Conjugated Hyperbilirubinemia
In patients with conjugated hyperbilirubinemia, the serum aminotransferases should be ordered for evidence of hepatocellular injury, alkaline phosphatase, and GGT levels for evidence of obstruction in biliary channels, prothrombin time/INR, and serum albumin to evaluate for hepatic constructed function. Additional tests like TORCH titers, urine cultures, viral cultures, serologic titers, Newborn screening results, specific tests for inborn errors of metabolism, alpha-1 antitrypsin phenotype, and specific genetics tests may be needed depending on the scenario.
Radiology is oftentimes necessary equally part of the workup of neonatal cholestasis. Hepatic ultrasonography may help identify sludging in the biliary tree, gallstones, inspissated bile, and choledochal cysts. Triangular cord sign seen on hepatic ultrasound has high sensitivity and almost 100% specificity for biliary atresia.[78] Hepatobiliary scintigraphy is another tool increasingly used in evaluating neonatal cholestasis. Decreased excretion of tracer 24 hours after introduction suggests obstacle and further helps in excluding nonobstructive causes of cholestasis.[90] Prior treatment with phenobarbitone has been shown to improve the sensitivity for this imaging. Finally, liver biopsy is ordinarily considered the golden standard for diagnosing neonatal cholestasis. Histopathological estimation by an experienced pathologist will help to identify the correct diagnosis in 90% to 95% of cases and may prevent unnecessary interventions in patients with intrahepatic cholestasis.[91]
Treatment / Management
Treatment of Unconjugated Hyperbilirubinemia
Phototherapy and exchange transfusion are the mainstay of treatment for patients with unconjugated hyperbilirubinemia.
Phototherapy
Phototherapy (PT) remains the commencement-line treatment for managing pathological unconjugated hyperbilirubinemia. PT is very effective in reducing TSB to safe levels and reduces the risk of bilirubin toxicity and the need for exchange transfusion. Phototherapy is started based on risk factors and the TSB levels on the bilirubin nomogram.[eight] However, guidelines on the indications for PT in preterm infants are defective, specially in the United States, considering of a lack of testify. As such well-nigh hospitals in the U.S have instituted their own guidelines for the utilize of phototherapy and exchange transfusion in preterm infants based on nascency weight or gestational age.[30] The efficacy of phototherapy depends on the dose and wavelength of light used as well as the surface surface area of the infant'due south body exposed to it. Increasing the dose of PT can be achieved past placing phototherapy units at the minimum safe distance from the infant and increasing the number of units used.
Bilirubin absorbs light optimally in the blue-green range (460 to 490 nm). PT works by inducing bilirubin photoisomerization and converting bilirubin into lumirubin, which is the rate-limiting pace for bilirubin excretion.[92] During phototherapy, the eyes of the newborn must be covered to avert retinal injury. Measures are necessary to betrayal maximum torso surface area to the calorie-free and avoid interruptions in PT. It is important to maintain adequate hydration and ensure normal urine output as most bilirubin is excreted in the urine as lumirubin. Subsequently phototherapy is discontinued, in that location is an increase in the total serum bilirubin level known as the" rebound bilirubin." The "rebound bilirubin" level is usually lower than the level at the initiation of phototherapy and usually does not require reinitiation of phototherapy.[93] PT has been considered relatively safety, but recent evidence points towards possible long-term side furnishings. Reported side-effects with PT employ include rash, dehydration, hypocalcemia, retinal impairment, hemolysis due to oxidative damage, delay in PDA closure in preterm infants, and allergic reactions.[94]
Few studies take also reported an increased incidence of solid organ tumors and non-lymphocytic leukemias in children treated with phototherapy.[95][96] The statuary baby syndrome is another commonly described phenomenon associated with PT and results in irregular pigmentation of the skin, mucous membranes, and urine. It is ordinarily seen in neonates with elevated serum conjugated bilirubin levels. The mechanism is non clear just appears to exist related to the accumulation of photoisomers of bilirubin and biliverdin deposition.[97][98]
Commutation Transfusion
Substitution transfusion (ET), the first successful handling ever used for jaundice, is currently the 2d-line treatment for severe unconjugated hyperbilirubinemia.[99] It is indicated when there is a failure of response to PT, or the initial TSB levels are in the exchange range based on the nomogram. ET rapidly removes bilirubin as well every bit hemolysis, causing antibodies from circulation. A double volume exchange blood transfusion (160 to 180 ml/kg) is performed, replacing the neonate'due south blood in aliquots with crossed-matched blood. Since most of the full body bilirubin lies in the extravascular compartment complications, TSB levels immediately following ET is virtually 60% of the pre-exchange level that later on increase to 70 to 80% of pre-exchange levels every bit a result of equilibrium with an extravascular moiety of bilirubin. During ET, vitals should be monitored closely, and TSB, CBC, serum calcium, glucose, and electrolytes need to exist checked following procedure. Complications of ET include electrolyte abnormalities like hypocalcemia and hyperkalemia, cardiac arrhythmias, thrombocytopenia, blood-borne infections, portal vein thrombosis, graft versus host disease, and necrotizing enterocolitis (NEC).[100][101] Phototherapy should resume after exchange transfusion until the bilirubin reaches a level where it can be safely discontinued.
Intravenous Immunoglobulin (IVIG)
IVIG is used when allowed-mediated hemolysis is the crusade of UHB jaundice and prevents RBC hemolysis by blanket Fc receptors on RBCs.The AAP recommends IVIG infusion in immune-mediated hemolysis if TSB remains within two to 3 mg/dl of exchange level despite intensive phototherapy.[102][103] However, the evidence that the employ of IVIG reduces the need for ET is not very clear. Notwithstanding, IVIG is often used in clinical exercise to manage unconjugated hyperbilirubinemia.
Treatment of Conjugated Hyperbilirubinemia
Treatment of conjugated hyperbilirubinemia is tailored to the specific etiology. Patients diagnosed with biliary atresia require a Kasai operation (hepatic portoenterostomy) preferably within ii months of life for best outcomes.[37] The Kasai operation involves removing the atretic biliary ducts and gristly plate and Roux-en-Y anastomosis of jejunum with the remaining ducts to provide an alternative pathway for biliary drainage.[104] Infectious causes of cholestasis would exist treated with specific anti-microbial, whereas handling with cholic acrid and chenodeoxycholic acid is often curative for many BASDs. Metabolic causes of cholestasis would typically respond to the improvement of the primary disorder and liver functions. Patients with GALD announced to answer well to IVIG and double volume exchange transfusion. Liver transplant, when available, is curative but is technically challenging in this historic period group.[58] Parenteral nutrition-induced cholestasis is managed with cyclic PN, reducing the duration of exposure and initiating enteral feeds as early as possible. Manganese and copper content of PN should be reduced to minimize liver injury.
Differential Diagnosis
The differential diagnosis for neonatal jaundice is quite limited as it can hands exist diagnosed past a physical exam in a newborn. In a rare situation, high carotene levels may cause yellowish discoloration of the skin and may exist mistaken to be hyperbilirubinemia.[34] At that place is, withal, no interest of the sclera or mucosa in carotenemia. Carotenemia arises from the ingestion of carotenoid-containing foods like carrots, mangos, light-green leafy vegetables, sweet potatoes, apricots, and melons, which is why information technology is unlikely that a newborn volition present with this. Still, as discussed in previous sections, the etiology of the two types of neonatal hyperbilirubinemia is quite extensive. Thorough cognition of these conditions is required for timely diagnosis and appropriate treatment.
Staging
Bilirubin encephalopathy in patients with severe unconjugated hyperbilirubinemia has different manifestations depending on the time of presentation. The level at which unconjugated bilirubin becomes neurotoxic is unclear, and kernicterus has been reported in infants in the absence of markedly elevated levels of bilirubin on autopsy.
Astute bilirubin encephalopathy: has been described to evolve through three stages:
Phase 1: The symptoms of phase 1 are seen during the offset one-ii days of illness and are marked by poor feeding, sluggishness, hypotonia, or frank seizures.
Stage ii: If the infants go on to deteriorate, they may progress to phase 2, characterized by increased tone, especially of the extensor group of muscles leading to opisthotonus and retrocollis. These signs are typically seen during the heart of the first week of illness.
Phase iii: This stage, seen later on the first calendar week, is mainly dominated by increased tone.
Chronic Bilirubin encephalopathy: This condition is present in two forms depending on the timing of symptoms.
Chronic Bilirubin encephalopathy in the Commencement year: These patients nowadays with hypotonia, exaggerated deep tendon reflexes, obligatory tonic neck reflexes, delayed motor milestones
Chronic Bilirubin encephalopathy beyond the Starting time year: Highlights of this phase include movement disorders (well-nigh commonly choreoathetosis), choreo-athetoid type of cognitive palsy, dental enamel hypoplasia, upward gaze abnormality, and sensorineural hearing loss.[72]
Prognosis
With treatment, the prognosis for almost types of unconjugated hyperbilirubinemia is excellent. In those with delayed or inadequate treatment, bilirubin encephalopathy may ensue. The brunt of bilirubin encephalopathy is significantly higher in developing and resources-limited nations.[68] Reports suggest a resurgence of kernicterus in countries where this complication had virtually disappeared in the past. This has been attributed mainly to the early discharge of newborns from the birthing hospital. Patients with Crigler-Najjar type one carry a poor prognosis and require liver transplantation for a definitive cure. In the absence of liver transplantation, bilirubin encephalopathy is common.
The prognosis for conjugated hyperbilirubinemia depends on the etiology. The outcome and prognosis of patients with biliary atresia are significantly improved by early diagnosis and surgery within threescore days of life. Similarly, patients with bile acid synthesis disorder (BASD) take an fantabulous prognosis as they respond very well to medical handling. Historically, the prognosis for gestational alloimmune liver illness (GALD) was poor, with up to 80% mortality without liver transplantation. However, with the advent of IVIG use and double volume exchange transfusion, the prognosis for this disease has greatly improved in contempo years.[105] The prognosis for most of the other types of cholestasis is oftentimes not very favorable, and many of these patients will require multidisciplinary interventions.
Complications
Newborns with severe hyperbilirubinemia are at risk for bilirubin-induced neurologic dysfunction (BIND). Bilirubin binds to globus pallidus, hippocampus, cerebellum, and subthalamic nuclear bodies, causing neurotoxicity.[106] Acutely, this manifests as acute bilirubin encephalopathy (ABE), characterized by lethargy, hypotonia, and decreased suck. At this phase, the affliction is reversible. However, if ABE were to progress, patients tin develop chronic bilirubin encephalopathy/kernicterus, which is then irreversible. It manifests every bit choreo-athetoid cerebral palsy, seizures, arching, posturing, gaze abnormality, and sensorineural hearing loss. Patients with neonatal cholestasis are at risk of developing liver failure, cirrhosis, and even hepatocellular carcinoma in a few cases. Long-continuing cholestasis may as well lead to failure to thrive and fat-soluble vitamin deficiencies.
Consultations
A pediatric or neonatal provider tin can manage about patients with unconjugated hyperbilirubinemia. Withal, patients suspected of genetic causes of hyperbilirubinemia may demand consultations and follow-ups with a pediatric gastroenterologist, hematologist, and medical geneticist.
Patients suspected of neonatal cholestasis should be referred to a pediatric gastroenterologist at the earliest. Most of these patients will need a battery of investigation, and once a cause of cholestasis is identified, more referrals would be warranted. Infants diagnosed with biliary atresia also demand a referral to a pediatric gastrointestinal surgeon for corrective surgery. Likewise, patients with inborn errors of metabolism would demand a consultation with a metabolic specialist as well as a medical geneticist and a Dietician experienced in metabolic disorders.
Deterrence and Patient Education
Detailed counseling, depending on the etiology of neonatal jaundice, is vital to improving the long-term outcome. Well-nigh patients with the common causes of unconjugated hyperbilirubinemia have an excellent prognosis, and parents need to be educated to convalesce fearfulness and anxiety. Jaundice from etiologies that conduct poor prognosis ofttimes requires multidisciplinary interventions, and parents should be adequately counseled and educated. Genetic counseling and referrals to medical geneticists should likewise be offered to parents whenever a child is diagnosed with hereditary hyperbilirubinemias.
Enhancing Healthcare Squad Outcomes
Neonatal jaundice is a mutual condition with varied etiologies. Near cases are benign with an excellent prognosis and resolve with or without treatment. However, bilirubin encephalopathy can complicate clinical form in a few. Health care professionals taking care of newborn needs to be aware of this. While many weather condition that cause jaundice cannot be diagnosed right away, education almost the affliction is critical. Nurses and parents are oftentimes the get-go to discover jaundice in a newborn. After discharge from the birth hospital, parents demand to be educated by the nurses, pediatricians, obstetricians, and the family practice providers to monitor for jaundice and seek medical care if it worsens.
The availability of a 2-color icterometer can help parents place jaundice earlier for prompt medical intervention. Nurses tin can too train mothers on how to examine the pare and eyes of neonates for jaundice. In addition, a smartphone app can also assist parents assess jaundice. An interprofessional team approach including nurses, lab-technician, providers from various sub-specialties, and nutritionists is necessary for the best outcome. Every health intendance provider involved in the care of a jaundiced newborn needs to be updated on current evidence-based direction approaches. Nurses play a vital role by monitoring treatments, educating parents, and keeping the squad apprised virtually changes in the patient's condition. [Level v] As per the American Academy of Pediatrics, every newborn must have a predischarge bilirubin cheque and should also exist assessed for run a risk factors associated with the development of severe hyperbilirubinemia to improve patient outcomes.[8] [Level 3]
Review Questions
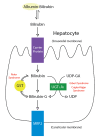
Figure
Metabolic pathway for bilirubin in the hepatocyte. Bilirubin-G corresponds to bilirubin glucuronate, where the donor is uridine diphosphate glucuronic acrid (UDP-GA). This is catalyzed by the enzyme uridine diphosphate-glucuronyltransferase (UGT1A1). Gilbert (more...)
References
- 1.
-
Gale R, Seidman DS, Stevenson DK. Hyperbilirubinemia and early belch. J Perinatol. 2001 January-Feb;21(ane):40-iii. [PubMed: 11268867]
- 2.
-
Mitra Southward, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med (Lond). 2017 Dec 02;78(12):699-704. [PubMed: 29240507]
- 3.
-
Kaplan M, Renbaum P, Levy-Lahad E, Hammerman C, Lahad A, Beutler E. Gilbert syndrome and glucose-6-phosphate dehydrogenase deficiency: a dose-dependent genetic interaction crucial to neonatal hyperbilirubinemia. Proc Natl Acad Sci U South A. 1997 Oct 28;94(22):12128-32. [PMC free article: PMC23725] [PubMed: 9342374]
- four.
-
Moncrieff MW, Dunn J. Phototherapy for hyperbilirubinaemia in very depression birthweight infants. Arch Dis Child. 1976 February;51(two):124-6. [PMC gratuitous article: PMC1545885] [PubMed: 944021]
- 5.
-
Bhutani VK, Wong R. Bilirubin-induced neurologic dysfunction (Demark). Semin Fetal Neonatal Med. 2015 Feb;20(1):one. [PubMed: 25577656]
- 6.
-
Ip Southward, Chung M, Kulig J, O'Brien R, Sege R, Glicken S, Maisels MJ, Lau J., American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An prove-based review of important problems apropos neonatal hyperbilirubinemia. Pediatrics. 2004 Jul;114(1):e130-53. [PubMed: 15231986]
- 7.
-
Leung AK, Sauve RS. Breastfeeding and breast milk jaundice. J R Soc Health. 1989 Dec;109(6):213-7. [PubMed: 2513410]
- eight.
-
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn babe 35 or more weeks of gestation. Pediatrics. 2004 Jul;114(ane):297-316. [PubMed: 15231951]
- 9.
-
Shahid R, Graba Southward. Result and price analysis of implementing selective Coombs testing in the newborn nursery. J Perinatol. 2012 Dec;32(12):966-9. [PubMed: 22441112]
- 10.
-
Desjardins 50, Blajchman MA, Chintu C, Gent Yard, Zipursky A. The spectrum of ABO hemolytic affliction of the newborn infant. J Pediatr. 1979 Sep;95(3):447-9. [PubMed: 469673]
- xi.
-
ACOG practice bulletin. Prevention of Rh D alloimmunization. Number 4, May 1999 (replaces educational bulletin Number 147, October 1990). Clinical management guidelines for obstetrician-gynecologists. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 1999 Jul;66(1):63-70. [PubMed: 10458556]
- 12.
-
Gómez-Manzo Due south, Marcial-Quino J, Vanoye-Carlo A, Serrano-Posada H, Ortega-Cuellar D, González-Valdez A, Castillo-Rodríguez RA, Hernández-Ochoa B, Sierra-Palacios E, Rodríguez-Bustamante E, Arreguin-Espinosa R. Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. Int J Mol Sci. 2016 Dec 09;17(12) [PMC complimentary commodity: PMC5187869] [PubMed: 27941691]
- 13.
-
Grace RF, Zanella A, Neufeld EJ, Morton DH, Eber S, Yaish H, Glader B. Erythrocyte pyruvate kinase deficiency: 2015 status report. Am J Hematol. 2015 Sep;90(nine):825-xxx. [PMC free article: PMC5053227] [PubMed: 26087744]
- fourteen.
-
Da Costa L, Galimand J, Fenneteau O, Mohandas N. Hereditary spherocytosis, elliptocytosis, and other red prison cell membrane disorders. Blood Rev. 2013 Jul;27(iv):167-78. [PubMed: 23664421]
- 15.
-
Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008 October xviii;372(9647):1411-26. [PubMed: 18940465]
- 16.
-
Gallagher PG, Weed SA, Tse WT, Benoit L, Morrow JS, Marchesi SL, Mohandas N, Forget BG. Recurrent fatal hydrops fetalis associated with a nucleotide substitution in the erythrocyte beta-spectrin gene. J Clin Invest. 1995 Mar;95(iii):1174-82. [PMC free article: PMC441455] [PubMed: 7883966]
- 17.
-
McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013 Jul 11;(7):CD004074. [PMC costless article: PMC6544813] [PubMed: 23843134]
- 18.
-
Fogarty One thousand, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, Simes J, Tarnow-Mordi W. Delayed vs early on umbilical cord clamping for preterm infants: a systematic review and meta-assay. Am J Obstet Gynecol. 2018 January;218(1):1-18. [PubMed: 29097178]
- 19.
-
Fenton C, McNinch NL, Bieda A, Dowling D, Damato E. Clinical Outcomes in Preterm Infants Following Establishment of a Delayed Umbilical Cord Clamping Exercise Alter. Adv Neonatal Care. 2018 Jun;18(iii):223-231. [PubMed: 29794839]
- 20.
-
Nakagawa M, Ishida Y, Nagaoki Y, Ohta H, Shimabukuro R, Hirata K, Yamanaka M, Kusakawa I. Correlation between umbilical cord hemoglobin and rate of jaundice requiring phototherapy in good for you newborns. Pediatr Int. 2015 Aug;57(4):626-8. [PubMed: 25533043]
- 21.
-
Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995 November 02;333(18):1171-5. [PubMed: 7565971]
- 22.
-
Anderson NB, Calkins KL. Neonatal Indirect Hyperbilirubinemia. Neoreviews. 2020 Nov;21(xi):e749-e760. [PubMed: 33139512]
- 23.
-
Maruo Y, Nakahara S, Yanagi T, Nomura A, Mimura Y, Matsui K, Sato H, Takeuchi Y. Genotype of UGT1A1 and phenotype correlation between Crigler-Najjar syndrome blazon II and Gilbert syndrome. J Gastroenterol Hepatol. 2016 Feb;31(two):403-8. [PubMed: 26250421]
- 24.
-
Grunebaum Eastward, Amir J, Merlob P, Mimouni M, Varsano I. Chest mild jaundice: natural history, familial incidence and late neurodevelopmental issue of the infant. Eur J Pediatr. 1991 Feb;150(four):267-70. [PubMed: 2029918]
- 25.
-
Preer GL, Philipp BL. Understanding and managing breast milk jaundice. Arch Dis Child Fetal Neonatal Ed. 2011 Nov;96(6):F461-6. [PubMed: 20688866]
- 26.
-
Rubarth LB. Infants of diabetic mothers. Neonatal Netw. 2013 Nov-Dec;32(6):416-8. [PubMed: 24195802]
- 27.
-
Amin SB. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol. 2004 Oct;28(5):340-vii. [PubMed: 15686265]
- 28.
-
Maisels MJ, Kring E. Gamble of sepsis in newborns with astringent hyperbilirubinemia. Pediatrics. 1992 Nov;90(five):741-3. [PubMed: 1408547]
- 29.
-
Hansen TW. Therapeutic approaches to neonatal jaundice: an international survey. Clin Pediatr (Phila). 1996 Jun;35(six):309-sixteen. [PubMed: 8782955]
- xxx.
-
Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An arroyo to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol. 2012 Sep;32(nine):660-4. [PubMed: 22678141]
- 31.
-
McDonagh AF. Is bilirubin good for you? Clin Perinatol. 1990 Jun;17(two):359-69. [PubMed: 2196134]
- 32.
-
Hegyi T, Goldie E, Hiatt M. The protective function of bilirubin in oxygen-radical diseases of the preterm baby. J Perinatol. 1994 Jul-Aug;fourteen(iv):296-300. [PubMed: 7965225]
- 33.
-
Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, McLin VA, Molleston JP, Neimark E, Ng VL, Karpen SJ. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the Due north American Society for Pediatric Gastroenterology, Hepatology, and Diet and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017 Jan;64(one):154-168. [PubMed: 27429428]
- 34.
-
Pan DH, Rivas Y. Jaundice: Newborn to Age 2 Months. Pediatr Rev. 2017 Nov;38(11):499-510. [PubMed: 29093118]
- 35.
-
The NS, Honein MA, Caton AR, Moore CA, Siega-Riz AM, Druschel CM., National Birth Defects Prevention Written report. Run a risk factors for isolated biliary atresia, National Nativity Defects Prevention Study, 1997-2002. Am J Med Genet A. 2007 Oct 01;143A(19):2274-84. [PubMed: 17726689]
- 36.
-
Takamizawa S, Zaima A, Muraji T, Kanegawa M, Akasaka Y, Satoh South, Nishijima E. Can biliary atresia be diagnosed by ultrasonography alone? J Pediatr Surg. 2007 Dec;42(12):2093-half dozen. [PubMed: 18082715]
- 37.
-
Serinet MO, Wildhaber Be, Broué P, Lachaux A, Sarles J, Jacquemin E, Gauthier F, Chardot C. Affect of age at Kasai performance on its results in late childhood and boyhood: a rational basis for biliary atresia screening. Pediatrics. 2009 May;123(v):1280-6. [PubMed: 19403492]
- 38.
-
Balistreri WF, Bezerra JA. Whatsoever happened to "neonatal hepatitis"? Clin Liver Dis. 2006 Feb;10(1):27-53, v. [PubMed: 16376793]
- 39.
-
Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, Pawlik TM. Choledochal cysts: presentation, clinical differentiation, and direction. J Am Coll Surg. 2014 Dec;219(6):1167-80. [PMC free article: PMC4332770] [PubMed: 25442379]
- xl.
-
Amedee-Manesme O, Bernard O, Brunelle F, Hadchouel M, Polonovski C, Baudon JJ, Beguet P, Alagille D. Sclerosing cholangitis with neonatal onset. J Pediatr. 1987 Aug;111(ii):225-9. [PubMed: 3612394]
- 41.
-
Ljung R, Ivarsson S, Nilsson P, Solvig J, Wattsgård C, Borulf S. Cholelithiasis during the commencement year of life: case reports and literature review. Acta Paediatr. 1992 January;81(1):69-72. [PubMed: 1600308]
- 42.
-
Plosa EJ, Esbenshade JC, Fuller MP, Weitkamp JH. Cytomegalovirus infection. Pediatr Rev. 2012 Apr;33(4):156-63; quiz 163. [PubMed: 22474112]
- 43.
-
Roelofsen H, van der Veere CN, Ottenhoff R, Schoemaker B, Jansen PL, Oude Elferink RP. Decreased bilirubin ship in the perfused liver of endotoxemic rats. Gastroenterology. 1994 Oct;107(4):1075-84. [PubMed: 7926459]
- 44.
-
Jesina D. Alagille Syndrome: An Overview. Neonatal Netw. 2017 Nov 01;36(6):343-347. [PubMed: 29185945]
- 45.
-
Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999 Mar;29(3):822-9. [PubMed: 10051485]
- 46.
-
Li L, Wang NL, Gong JY, Wang JS. [Infantile cholestasis caused by CFTR mutation: case study and literature review]. Zhonghua Er Ke Za Zhi. 2016 Nov 02;54(eleven):851-855. [PubMed: 27806795]
- 47.
-
Townsend South, Newsome P, Turner AM. Presentation and prognosis of liver disease in alpha-1 antitrypsin deficiency. Expert Rev Gastroenterol Hepatol. 2018 Aug;12(8):745-747. [PubMed: 29768056]
- 48.
-
Karadag N, Zenciroglu A, Eminoglu FT, Dilli D, Karagol BS, Kundak A, Dursun A, Hakan N, Okumus Due north. Literature review and outcome of classic galactosemia diagnosed in the neonatal period. Clin Lab. 2013;59(9-10):1139-46. [PubMed: 24273939]
- 49.
-
Chinsky JM, Singh R, Ficicioglu C, van Karnebeek CDM, Grompe M, Mitchell G, Waisbren SE, Gucsavas-Calikoglu K, Wasserstein MP, Coakley K, Scott CR. Diagnosis and treatment of tyrosinemia blazon I: a US and Canadian consensus group review and recommendations. Genet Med. 2017 December;nineteen(12) [PMC free article: PMC5729346] [PubMed: 28771246]
- l.
-
Jacquemin E. Progressive familial intrahepatic cholestasis. Genetic basis and treatment. Clin Liver Dis. 2000 Nov;four(4):753-63. [PubMed: 11232355]
- 51.
-
Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009 Jan 08;iv:1. [PMC costless article: PMC2647530] [PubMed: 19133130]
- 52.
-
Balderdash LN, Roche E, Song EJ, Pedersen J, Knisely AS, van Der Hagen CB, Eiklid K, Aagenaes O, Freimer NB. Mapping of the locus for cholestasis-lymphedema syndrome (Aagenaes syndrome) to a 6.6-cM interval on chromosome 15q. Am J Hum Genet. 2000 Oct;67(four):994-9. [PMC free commodity: PMC1287903] [PubMed: 10968776]
- 53.
-
Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best Pract Res Clin Gastroenterol. 2010 Oct;24(5):555-71. [PubMed: 20955959]
- 54.
-
Lauriti G, Zani A, Aufieri R, Cananzi Grand, Chiesa PL, Eaton S, Pierro A. Incidence, prevention, and handling of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr. 2014 Jan;38(1):70-85. [PubMed: 23894170]
- 55.
-
Buchman AL, Iyer Grand, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006 Jan;43(1):nine-nineteen. [PubMed: 16374841]
- 56.
-
Duerksen DR, Van Aerde JE, Chan Yard, Thomson AB, Jewell LJ, Clandinin MT. Total parenteral nutrition impairs bile menstruation and alters bile composition in newborn piglet. Dig Dis Sci. 1996 Sep;41(9):1864-70. [PubMed: 8794808]
- 57.
-
Pan X, Kelly S, Melin-Aldana H, Malladi P, Whitington PF. Novel mechanism of fetal hepatocyte injury in congenital alloimmune hepatitis involves the final complement cascade. Hepatology. 2010 Jun;51(6):2061-8. [PubMed: 20512994]
- 58.
-
Feldman AG, Whitington PF. Neonatal hemochromatosis. J Clin Exp Hepatol. 2013 Dec;three(iv):313-20. [PMC free article: PMC3940210] [PubMed: 25755519]
- 59.
-
Bhutani VK. Editorial: building prove to manage newborn jaundice worldwide. Indian J Pediatr. 2012 Feb;79(ii):253-5. [PubMed: 22183759]
- 60.
-
Alkhotani A, Eldin EE, Zaghloul A, Mujahid S. Evaluation of neonatal jaundice in the Makkah region. Sci Rep. 2014 Apr 25;4:4802. [PMC gratuitous article: PMC3999454] [PubMed: 24763104]
- 61.
-
Winfield CR, MacFaul R. Clinical study of prolonged jaundice in chest- and bottle-fed babies. Arch Dis Kid. 1978 Jun;53(vi):506-seven. [PMC free article: PMC1544945] [PubMed: 686778]
- 62.
-
Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006 Sep 12;175(vi):587-ninety. [PMC free article: PMC1559442] [PubMed: 16966660]
- 63.
-
Ding Yard, Zhang South, Yao D, Na Q, Wang H, Li L, Yang L, Huang Westward, Wang Y, Xu J. An epidemiological survey on neonatal jaundice in China. Mentum Med J (Engl). 2001 Apr;114(iv):344-seven. [PubMed: 11780450]
- 64.
-
Bhutani VK, Zipursky A, Blencowe H, Khanna R, Sgro 1000, Ebbesen F, Bell J, Mori R, Slusher TM, Fahmy N, Paul VK, Du Fifty, Okolo AA, de Almeida MF, Olusanya BO, Kumar P, Cousens Southward, Lawn JE. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and damage estimates for 2010 at regional and global levels. Pediatr Res. 2013 Dec;74 Suppl one:86-100. [PMC free article: PMC3873706] [PubMed: 24366465]
- 65.
-
Moore LG, Newberry MA, Freeby GM, Crnic LS. Increased incidence of neonatal hyperbilirubinemia at 3,100 m in Colorado. Am J Dis Child. 1984 Feb;138(2):157-61. [PubMed: 6695871]
- 66.
-
Drew JH, Barrie J, Horacek I, Kitchen WH. Factors influencing jaundice in immigrant Greek infants. Arch Dis Child. 1978 Jan;53(ane):49-52. [PMC gratuitous article: PMC1544832] [PubMed: 626518]
- 67.
-
Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks' gestation) - Summary. Paediatr Kid Health. 2007 May;12(five):401-18. [PMC free article: PMC2528724] [PubMed: 19030400]
- 68.
-
Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. Northward Engl J Med. 2013 Nov 21;369(21):2021-xxx. [PubMed: 24256380]
- 69.
-
Dick MC, Mowat AP. Hepatitis syndrome in infancy--an epidemiological survey with ten year follow up. Arch Dis Child. 1985 Jun;60(half dozen):512-half dozen. [PMC free article: PMC1777358] [PubMed: 3874604]
- 70.
-
Gottesman LE, Del Vecchio MT, Aronoff SC. Etiologies of conjugated hyperbilirubinemia in infancy: a systematic review of 1692 subjects. BMC Pediatr. 2015 November xx;fifteen:192. [PMC gratuitous article: PMC4654877] [PubMed: 26589959]
- 71.
-
D'Alessandro AM, Knechtle SJ, Chin LT, Fernandez LA, Yagci Grand, Leverson G, Kalayoglu 1000. Liver transplantation in pediatric patients: xx years of experience at the University of Wisconsin. Pediatr Transplant. 2007 Sep;11(half dozen):661-70. [PubMed: 17663691]
- 72.
-
Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med. 2001 Feb 22;344(8):581-90. [PubMed: 11207355]
- 73.
-
Poland RL, Odell GB. Physiologic jaundice: the enterohepatic circulation of bilirubin. N Engl J Med. 1971 Jan 07;284(1):1-half dozen. [PubMed: 4922346]
- 74.
-
Brouillard RP. Measurement of carmine blood cell life-span. JAMA. 1974 December 02;230(9):1304-five. [PubMed: 4479604]
- 75.
-
Chuniaud Fifty, Dessante M, Chantoux F, Blondeau JP, Francon J, Trivin F. Cytotoxicity of bilirubin for homo fibroblasts and rat astrocytes in culture. Result of the ratio of bilirubin to serum albumin. Clin Chim Acta. 1996 Dec thirty;256(two):103-fourteen. [PubMed: 9027422]
- 76.
-
Amato MM, Kilguss NV, Gelardi NL, Cashore WJ. Dose-effect relationship of bilirubin on striatal synaptosomes in rats. Biol Neonate. 1994;66(5):288-93. [PubMed: 7873694]
- 77.
-
Hoffman DJ, Zanelli SA, Kubin J, Mishra OP, Delivoria-Papadopoulos M. The in vivo outcome of bilirubin on the North-methyl-D-aspartate receptor/ion channel complex in the brains of newborn piglets. Pediatr Res. 1996 Dec;40(six):804-eight. [PubMed: 8947954]
- 78.
-
Benchimol EI, Walsh CM, Ling SC. Early diagnosis of neonatal cholestatic jaundice: test at 2 weeks. Tin can Fam Physician. 2009 Dec;55(12):1184-92. [PMC costless article: PMC2793221] [PubMed: 20008595]
- 79.
-
Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. Due north Engl J Med. 1998 October 22;339(17):1217-27. [PubMed: 9780343]
- fourscore.
-
Chen HL, Wu SH, Hsu SH, Liou BY, Chen HL, Chang MH. Jaundice revisited: contempo advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018 Oct 26;25(1):75. [PMC costless article: PMC6203212] [PubMed: 30367658]
- 81.
-
Hamza A. Kernicterus. Autops Case Rep. 2019 Jan-Mar;9(ane):e2018057. [PMC gratuitous article: PMC6394357] [PubMed: 30863731]
- 82.
-
Vij M, Rela Yard. Biliary atresia: pathology, etiology and pathogenesis. Time to come Sci OA. 2020 Mar 17;6(v):FSO466. [PMC gratuitous article: PMC7273417] [PubMed: 32518681]
- 83.
-
Matthai J, Paul S. Evaluation of cholestatic jaundice in immature infants. Indian Pediatr. 2001 Aug;38(8):893-8. [PubMed: 11521001]
- 84.
-
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks' gestation: an update with clarifications. Pediatrics. 2009 Oct;124(4):1193-viii. [PubMed: 19786452]
- 85.
-
Johnson L, Bhutani VK. Guidelines for management of the jaundiced term and about-term infant. Clin Perinatol. 1998 Sep;25(3):555-74, viii. [PubMed: 9779334]
- 86.
-
Wainer S, Rabi Y, Parmar SM, Allegro D, Lyon Thousand. Touch of skin tone on the functioning of a transcutaneous jaundice meter. Acta Paediatr. 2009 December;98(12):1909-15. [PubMed: 19764923]
- 87.
-
Casnocha Lucanova 50, Matasova 1000, Zibolen Chiliad, Krcho P. Accuracy of transcutaneous bilirubin measurement in newborns afterwards phototherapy. J Perinatol. 2016 Oct;36(10):858-61. [PubMed: 27279078]
- 88.
-
Hulzebos CV, Dijk PH, van Imhoff DE, Bos AF, Lopriore Eastward, Offringa M, Ruiter SA, van Braeckel KN, Krabbe PF, Quik EH, van Toledo-Eppinga Fifty, Nuytemans DH, van Wassenaer-Leemhuis AG, Benders MJ, Korbeeck-van Hof KK, van Lingen RA, Groot Jebbink LJ, Liem D, Mansvelt P, Buijs J, Govaert P, van Vliet I, Mulder TL, Wolfs C, Fetter WP, Laarman C., BARTrial Study Grouping. The bilirubin albumin ratio in the direction of hyperbilirubinemia in preterm infants to amend neurodevelopmental outcome: a randomized controlled trial--BARTrial. PLoS One. 2014;9(six):e99466. [PMC costless article: PMC4057208] [PubMed: 24927259]
- 89.
-
Steinborn M, Seelos KC, Heuck A, von Voss H, Reiser M. MR findings in a patient with Kernicterus. Eur Radiol. 1999;nine(9):1913-5. [PubMed: 10602975]
- xc.
-
McKiernan PJ, Bakery AJ, Kelly DA. The frequency and outcome of biliary atresia in the Uk and Ireland. Lancet. 2000 January 01;355(9197):25-ix. [PubMed: 10615887]
- 91.
-
Morotti RA, Jain D. Pediatric Cholestatic Disorders: Approach to Pathologic Diagnosis. Surg Pathol Clin. 2013 Jun;vi(two):205-25. [PubMed: 26838972]
- 92.
-
Bhutani VK., Commission on Fetus and Newborn. American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn baby 35 or more weeks of gestation. Pediatrics. 2011 Oct;128(4):e1046-52. [PubMed: 21949150]
- 93.
-
Yetman RJ, Parks DK, Huseby V, Mistry K, Garcia J. Rebound bilirubin levels in infants receiving phototherapy. J Pediatr. 1998 Nov;133(five):705-7. [PubMed: 9821435]
- 94.
-
Wang J, Guo Grand, Li A, Cai WQ, Wang Ten. Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp Ther Med. 2021 Mar;21(iii):231. [PMC costless commodity: PMC7859475] [PubMed: 33613704]
- 95.
-
Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective Cohort Study of Phototherapy and Babyhood Cancer in Northern California. Pediatrics. 2016 Jun;137(6) [PubMed: 27217477]
- 96.
-
Auger N, Laverdière C, Ayoub A, Lo East, Luu TM. Neonatal phototherapy and hereafter take a chance of childhood cancer. Int J Cancer. 2019 Oct xv;145(8):2061-2069. [PubMed: 30684392]
- 97.
-
Itoh S, Okada H, Kuboi T, Kusaka T. Phototherapy for neonatal hyperbilirubinemia. Pediatr Int. 2017 Sep;59(nine):959-966. [PubMed: 28563973]
- 98.
-
Kar South, Mohankar A, Krishnan A. Bronze babe syndrome. Indian Pediatr. 2013 Jun 08;l(half-dozen):624. [PubMed: 23942414]
- 99.
-
DIAMOND LK, ALLEN FH, THOMAS WO. Erythroblastosis fetalis. Seven. Handling with exchange transfusion. Northward Engl J Med. 1951 Jan 11;244(2):39-49. [PubMed: 14785788]
- 100.
-
Jackson JC. Agin events associated with exchange transfusion in healthy and ill newborns. Pediatrics. 1997 May;99(5):E7. [PubMed: 9113964]
- 101.
-
Patra Thou, Storfer-Isser A, Siner B, Moore J, Hack Grand. Adverse events associated with neonatal exchange transfusion in the 1990s. J Pediatr. 2004 May;144(5):626-31. [PubMed: 15126997]
- 102.
-
Alpay F, Sarici SU, Okutan Five, Erdem G, Ozcan O, Gökçay Due east. High-dose intravenous immunoglobulin therapy in neonatal immune haemolytic jaundice. Acta Paediatr. 1999 February;88(2):216-9. [PubMed: 10102158]
- 103.
-
Gottstein R, Cooke RW. Systematic review of intravenous immunoglobulin in haemolytic illness of the newborn. Arch Dis Kid Fetal Neonatal Ed. 2003 Jan;88(1):F6-x. [PMC gratuitous article: PMC1755998] [PubMed: 12496219]
- 104.
-
Ohi R. Surgery for biliary atresia. Liver. 2001 Jun;21(3):175-82. [PubMed: 11422780]
- 105.
-
Rand EB, Karpen SJ, Kelly S, Mack CL, Malatack JJ, Sokol RJ, Whitington PF. Handling of neonatal hemochromatosis with exchange transfusion and intravenous immunoglobulin. J Pediatr. 2009 Oct;155(iv):566-71. [PubMed: 19560784]
- 106.
-
Hankø Due east, Hansen TW, Almaas R, Lindstad J, Rootwelt T. Bilirubin induces apoptosis and necrosis in homo NT2-Northward neurons. Pediatr Res. 2005 February;57(2):179-84. [PubMed: 15611354]
Source: https://www.ncbi.nlm.nih.gov/books/NBK532930/
Post a Comment for "The Most Common Cause of Pathologic Hyperbilurubinemia in a Baby Is"